|
|
ICP-Atomic Emission Spectroscopy (ICP-AES)
 Kindly download Material Safety Data sheet and submit the hardcopy of filled sheet along with your sample Kindly download Material Safety Data sheet and submit the hardcopy of filled sheet along with your sample
|
|
| Make |
: SPECTRO Analytical Instruments GmbH, Germany |
| Model |
: ARCOS, Simultaneous ICP Spectrometer
|
| Specification |
:
(a) R.F. Generator: Maximum of 1.6 KW, 27.12 MHz
(b) Plasma: Radial plasma, having capability to analyse aqueous solutions with high dissolved solid content even up to 30 wt %. Aqueous solutions can be acidic, basic or neutral.
(c) Spectrometer: Wavelength Range: 130 nm to 770 nm, Resolution: approx. 9 pico meter, having capability to scan full spectrum to have qualitative information about the content of the sample.
(d). Detector: Charge Coupled Devices (CCD)
(e) Vertical Torch assembly having fully demountable quartz torch with individual tubes as well as a Ceramic Fully demountable torch for HF based solutions.
(f) Nebulizers: Concentric, cross flow, organic nebulizer (hydrocarbons, solvents) as well as cross flow nebulizer for HF containing solutions.
(g) Spray Chambers: HF Resistant Cyclonic Chamber and hydrocarbon solution spray chamber. Spray chambers suitable for cross flow Nebulizers are available./td>
|
|
 |
|
|
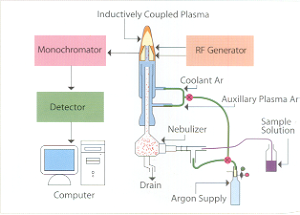
|
Inductively Coupled Plasma - Atomic Emission Spectrometry (ICP- AES) is an emission spectrophotometric technique, exploiting the fact that excited electrons emit energy at a given wavelength as they return to ground state after excitation by high temperature Argon Plasma. The fundamental characteristic of this process is that each element emits energy at specific wavelengths peculiar to its atomic character. The energy transfer for electrons when they fall back to ground state is unique to each element as it depends upon the electronic configuration of the orbital. The energy transfer is inversely proportional to the wavelength of electromagnetic radiation,
E = hc/ λ ... (where h is Planck's constant, c the velocity of light and λ is wavelength), and hence the wavelength of light emitted is also unique.
Although each element emits energy at multiple wavelengths, in the ICP-AES technique it is most common to select a single wavelength (or a very few) for a given element. The intensity of the energy emitted at the chosen wavelength is proportional to the amount (concentration) of that element in the sample being analyzed. Thus, by determining which wavelengths are emitted by a sample and by determining their intensities, the analyst can qualitatively and quantitatively find the elements from the given sample relative to a reference standard.
The wavelengths used in AES ranges from the upper part of the vacuum ultraviolet (160 nm) to the limit of visible light (800 nm). As borosilicate glass absorbs light below 310 nm and oxygen in air absorbs light below 200 nm, optical lenses and prisms are generally fabricated from quartz glass and optical paths are evacuated or filled by a non absorbing gas such as Argon.
|
|
|
 Precious metal estimation at low level Precious metal estimation at low level
 Heavy metal estimation at sub ppm level Heavy metal estimation at sub ppm level
 Rock, Soil, Fly ash (Complete analysis) Rock, Soil, Fly ash (Complete analysis)
 Environmental sample analysis (Air, Water, Soil, sediments, etc.) Environmental sample analysis (Air, Water, Soil, sediments, etc.)
 Biological samples (Urine, tooth, bone, etc.) Biological samples (Urine, tooth, bone, etc.)
 Polymer industries Polymer industries
 Pharmaceutical industries Pharmaceutical industries
|
Contact : 022-21596897
Email Id : icpaes@iitb.ac.in
|
1.Rocks, soils, ores and sediments can be processed using any one of the following procedures depending upon the requirements of the users and available facilities. a) Lithium Metaborate Fusion : 0.2 gm of sample and 0.6 gm lithium metaborate taken in a platinum crucible are heated on a burner till the mixture in the crucible turns into a glassy mass. The crucible is then placed in 30% nitric acid solution and stirred till all the glassy mass dissolves (usually two hours). The solution is then diluted to standard volume with distilled water. b) Hydrofluoric Acid Method : 0.5 gm of the sample is taken in a Teflon beaker and 10 ml nitric acid or perchloric acid and 5 ml Hydrofluoric acid are added to it. The solution is heated on a hot plate to dryness. 10 ml of aqua-regia is added to the dry mass and heated till everything dissolves. The solution is diluted to standard volume, 100 ml, with distilled water. Estimation of Silicon is not possible as silica is lost to the atmosphere as silicon tetrafluoride.
2. Analysis of air samples can be done and the process of sample preparation is as follows: Air is drawn in at a constant rate through a filter (usually made of cellulose ester or glass fiber). The filter is then digested by following methods: a) For cellulose ester filter - A weighed filter paper is taken in a beaker, 10 ml nitric acid added and heated on a hot plate. While still hot, perchloric acid is added dropwise till the organic matter is destroyed. The sample is concentrated by further heating and solution diluted with distilled water. b) For glass fibre filters - A weighed filter is taken in a teflon beaker, 5 ml of HF and 10 ml nitric acid added and heated on a hot plate. A few drops of perchloric acid are added and the solution concentrated to dryness. The residue is dissolved in 10 ml of nitric acid and diluted with distilled water.
3. Water samples can be analysed as such for elements like Ca, Mg, Mn, Fe, Zn, Na, K. But other minor elements need concentration. 500 ml of solution is concentrated to 50 ml with warming at 90o C. Volatile elements like mercury may get lost at higher temperature.
4. Organic samples (Blood Urine, Polymer etc.) : 10 ml of sample in 10 ml of nitric acid is heated on a hot plate and hot perchloric acid is added dropwise till all the organic matter is destroyed and solution becomes clear. Dilution is done with distilled water to desired volume.
5. Samples of metals and alloys can be digested using only nitric acid or hydrochloric acid or the combination of the two (aqua-regia). Crystalline samples that are water soluble can be submitted in their water dissolved form. Organic solvents cannot be accepted, because plasma cannot be sustained in presence of organic solvent. Hence the organic solvent has to be either volatilised or destroyed.
6. A minimum of 25 ml of solution is required for estimation of all elements.
|
|
Additional Instructions for users
|
1.Preferably sample should be submitted in solution form specifying the elements to be estimated and their approximate concentrations expected.
2.Generally 10 ml of solution is sufficient for estimation of about 10 to 15 elements
3.For special samples like rocks / ore samples, appropriate standards along with the samples should be submitted by user.
4.Explosive, poisonous samples and samples giving rise to toxic gases/fumes cannot be undertaken for ICP-AES analysis.
5.There is no upper limit for the number of samples acceptable for ICP-AES laboratory.
6.The nebulizer of the instrument is made out of glass material, hence samples should not have HF in the solution. Excess HF should be evaporated completely or may be neutralised using Boric Acid.
7.For estimation of silica in rock sample following method of solution preparation is generally used:
(a) 0.5 gram of rock powder + 1.5 gram of Lithium Meta-Borate is fused in a platinum crucible. After the fusion is complete the crucible is kept on a magnetic stirrer with 30% HNO3 in it, till the whole thing goes in solution. Same method should be followed for preparation of standard solutions.
(b) 0.5 gram of rock sample + HF + HNO3 is heated slowly in a teflon beaker. On digestion evaporate HF (in which case SiO2 may be lost) or neutralise HF with Boric acid.
(c) For some of the rocks HClO4 and HNO3 mixture can be used along with HF. One should remember that same method should be used for preparation of standard as well as blank.
Turbid solutions and highly viscous samples will not be entertained.
8.For biological samples organic matter should be destroyed using any of the following methods of solution preparation :
(a) A known quantity of sample is put in a Quartz/silica crucible and kept in an incinerator at 800o to 900oC. The ash is then dissolved in aqua-regia and diluted to a known volume using distilled water.
(b) A known quantity of sample is taken in a beaker, HNO3 added to it and heated. When it starts boiling HClO4 is added to it drop-wise and heating continued till all the organic matter is destroyed. The solution is then diluted to a know volume with distilled water.
9.Blank solution for each batch/set of samples should be provided.
|
1.Thompson M. and Walsh J.N. `Inductively Coupled Plasma Spectrometry', Blackie.(1989)
2.Moore G.L. `Introduction to Inductively Coupled Plasma', Elsevier.
|
|
ICP-AES Charges includes GST
|
| | Industry | University | National Lab/R&D's | IIT Bombay Users |
| Standarization & Estimation | 1475/- | 295/- | 679/- | 125/- | Per Sample
|
| Estimation for Subsequent sample | 531/- | 106/- | 236/- | 45/- | Per Sample
| | Sample Preparation | 3540/- | 708/- | 1593/- | 300/- | Per Sample
| | Qualitative Scan | 5900/- | 1180/- | 2655/- | 500/- | Per Sample (Plus sample preparation charges extra.)
|
|
|
|

